Access Agilent eNewsletter April 2016

Eliminate conventional SEC roadblocks with Agilent AdvanceBio SEC columns
Linda Lloyd, Agilent Biocolumns Product Manager
Therapeutic proteins are a rapidly growing segment of the pharmaceutical market. The aggregation/degradation status of therapeutic proteins is a critical quality attribute. Quality control regulations for therapeutic proteins, such as ICH(Q6B), clearly state that aggregates must be resolved from the desired product and quantitated. Size exclusion chromatography (SEC) separates solutes depending on their size in solution and is the method of choice for the quantitation of protein aggregates.
Better resolution and shorter run times with Agilent AdvanceBio SEC
There are numerous roadblocks associated with conventional SEC that can hold up the development and characterization of therapeutic proteins. These roadblocks include the speed of separation and long run times. Standard high-resolution conditions, using 300 mm columns and a flow rate of 0.8 mL/min, can result in sample run times of 20 minutes or more. Extended run times can limit maximum throughput to three samples an hour. The requirement to monitor aggregate formation during biotherapeutic development, for example during clone selection or while optimizing fermentation conditions, results in numerous samples needing SEC analysis. Long run times greatly restrict the ability to analyze large numbers of samples, leading to sample backlogs and creating data bottlenecks.
In addition, proteins are prone to degradation and aggregation under stress conditions. It is vital that the mobile phase does not affect the sample composition. Therefore, aqueous mobile phases, neutral pH, and low levels of salt are preferred. Most published methods for SEC analysis of antibody-drug conjugates using an aqueous mobile phase reveal poor peak shapes and incomplete resolution. This problem is often resolved using organic modifiers, such as propanol. However, poor resolution of the addition of organic modifiers leads to uncertainty and drives the need for repeat sample analysis or method revalidation.
Agilent’s new AdvanceBio SEC biocolumn has been developed from scratch to overcome these roadblocks. The column contains unique 2.7 µm silica particles with bonding chemistry designed for maximum efficiency without the risk of clogging or shear degradation of samples. The unique manufacturing method controls pore size, structure, and volume. A hydrophilic polymeric coating is applied to the particles to ensure protein peaks that are sharp and resolved.
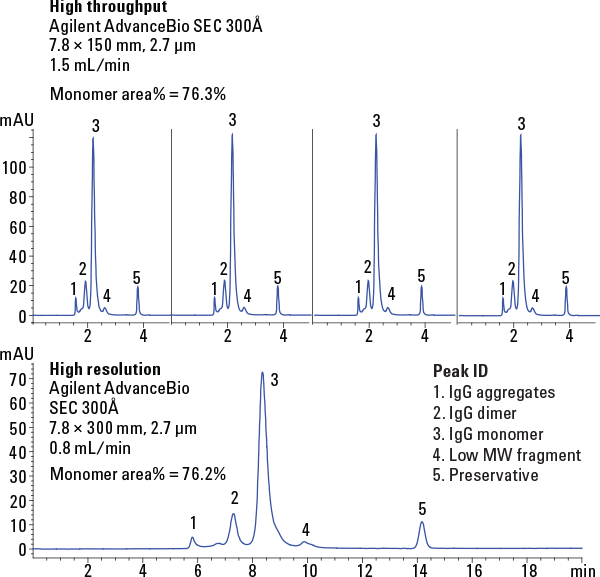
Figure 1. Comparative chromatograms of IgG under high-throughput and high-resolution conditions.
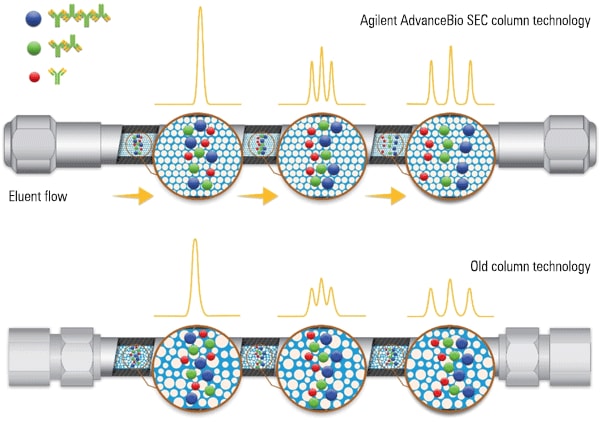
Figure 2. Agilent AdvanceBio SEC column in side-by-side technology comparison.
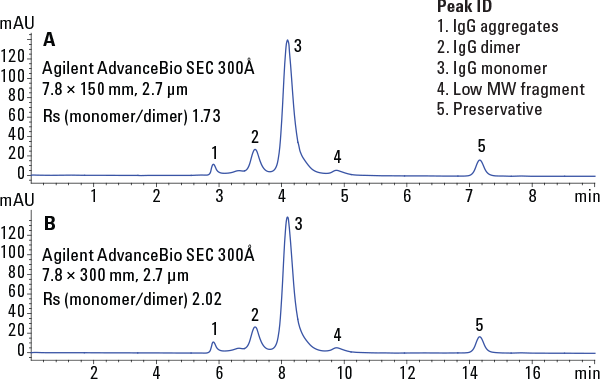
Figure 3. Size exclusion of immunoglobulin under high-resolution conditions.
New technology increases sample throughput and productivity by 400%
The breakthrough technology of the Agilent AdvanceBio SEC column eliminates slow separations allowing you to meet vital deadlines. The new silica particles provide the resolution to run shorter columns. AdvanceBio SEC columns achieved equivalent resolution in the separation of immunoglobulin G (IgG) on the shorter 150 mm column, as compared to the 300 mm column (Figure 1). The run time, however, is half as long using this shorter column. Furthermore, the excellent particle stability of the Agilent AdvanceBio SEC column allows operation at significantly higher flow rates without undue loss in performance.
The effect of increasing the flow rate to 1.5 mL/min, during the analysis of IgG on an Agilent AdvanceBio SEC 300Å 150 mm column is illustrated in Figure 2. The increased flow rate did not change quantitation of the monomer peak area or aggregate content determination. However, sample run times were reduced to 4.8 minutes at this higher flow rate of 1.5 mL/min. Our example shows the use of shorter 150 mm columns and higher flow rates of 1.5 mL/min achieves significantly increased sample throughput. An optimal capacity of 12 samples per hour is achieved using the higher flow rate, as well as a 400% increase in sample throughput and productivity.
Sample integrity, reproducible results, and elimination
of re-analysis
The results described above illustrate the increased productivity that this technology delivers. Agilent AdvanceBio SEC columns offer excellent resolution and size separations over a wide range of sample types, without the need to add an organic modifier to the mobile phase. In fact, AdvanceBio SEC allows the use of conditions that promote the native structure of the protein. What’s more, these conditions reduce uncertainty of sample integrity, leading to reproducible results and eliminating the need for sample re-analysis.
Agilent AdvanceBio SEC columns offer a breakthrough technology removing the roadblocks associated with SEC, ending uncertainty, and allowing you to resolve protein aggregates and degradants with speed and confidence (Figure 3).
Full details of this work are freely available in Agilent publication 5991-6458EN.
Agilent offers a full suite of Liquid Chromatography solutions
Agilent Advance Bio SEC columns deliver reproducible results—column to column, batch to batch, and lab to lab. In addition to AdvanceBio SEC, Agilent offers biochemists a wide array of liquid chromatography solutions, columns, and supplies, including The Agilent 1260 Infinity Bio-inert Quaternary LC which sets new standards in performance, reliability, and robustness for analysis of bio-molecules. Take a moment to view our informative video and learn more about characterization of monoclonal antibodies today.
For Research Use Only. Not for use in diagnostic procedures.
This information is subject to change without notice.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – April 2016
All articles in this issue
 Analyze low-ppm levels of active sulfur compounds with inert sample path on the Agilent 490 Micro GC
Analyze low-ppm levels of active sulfur compounds with inert sample path on the Agilent 490 Micro GC Fast arsenic speciation for food and urine analysis with Agilent LC-ICP-QQQ
Fast arsenic speciation for food and urine analysis with Agilent LC-ICP-QQQ Simplify achiral-chiral analysis of warfarin metabolites using Agilent 2D-LC/MS solutions
Simplify achiral-chiral analysis of warfarin metabolites using Agilent 2D-LC/MS solutions Optimized detection of gaseous sulfur compounds using Agilent J&W DB-Sulfur SCD GC column and Inert Flow Path
Optimized detection of gaseous sulfur compounds using Agilent J&W DB-Sulfur SCD GC column and Inert Flow Path Oil-free laboratory vacuum eliminates oil mess and disposal costs
Oil-free laboratory vacuum eliminates oil mess and disposal costs Eliminate conventional SEC roadblocks with Agilent AdvanceBio SEC columns
Eliminate conventional SEC roadblocks with Agilent AdvanceBio SEC columns Fast, economical assessment of herbals with Agilent 1290 Infinity LC system and LC columns
Fast, economical assessment of herbals with Agilent 1290 Infinity LC system and LC columns Agilent 5977B GC/MSD with HES offers improved VOC detection limits
Agilent 5977B GC/MSD with HES offers improved VOC detection limits
Figure 1

Comparative chromatograms of IgG under high-throughput and high-resolution conditions.
Figure 2

Agilent AdvanceBio SEC column in side-by-side technology comparison.
Figure 3

Size exclusion of immunoglobulin under high-resolution conditions.