Access Agilent eNewsletter September 2016

High-resolution, efficient quantification of aggregates in protein therapeutics with Agilent 1290 Infinity II 2D-LC Solution
Patrick Cronan and Michael Kruger
Agilent Application Scientists
Timothy Rice
Agilent Global Product Manager
Protein biopharmaceuticals, such as monoclonal antibodies and recombinant proteins, are currently in widespread use. Protein therapeutics are far more complicated than small molecule drugs and represent a complex analytical challenge. An in-depth characterization of these molecules is required during the development and lifetime of these crucial pharmaceuticals.[1]
Protein A is an immunoglobulin-Fc (IgG) receptor found in the cell wall of Staphylococcus aureus. It has strong affinity for polyclonal and monoclonal IgGs such as human IgG 1, IgG 2, and IgG 4. This is in addition to IgGs from other species such as rabbits and some mouse IgGs. Immobilized Protein A is commonly used for preparative and process scale purifications of IgG. At the analytical scale, the Agilent Bio-Monolith Protein A HPLC column can be used for fast quantification and small-scale purification of IgGs in complex mixtures or pure samples.[1,2]
Size exclusion chromatography (SEC) is the method of choice for quantification of aggregates in biotherapeutic samples. Protein A capture followed by SEC for aggregation analysis yields more accurate characterization as purer sample is analyzed. With the Agilent 1290 Infinity II 2D-LC solution, this analysis can be done with one injection. Protein A capture is performed in the first dimension. With this method, eluted IgG is trapped in the multiple heart-cutting loops and eluted in the second dimension onto an Agilent AdvanceBio SEC sizing column for accurate aggregate analysis (Figure 1).

Figure 1. Accurate aggregate analysis was achieved with AdvanceBio SEC column.
Agilent 1260 Infinity II Bio-Inert LC sets the stage for rapid aggregate analysis
The 2D-LC solution used in this study comprised of an Agilent 1260 Infinity II Bio-Inert LC as the first-dimension system. The bio-inert LC was coupled through an Agilent InfinityLab 2D-LC Quick Change Valve—a multiple heart-cutting valve (with 12 factory-installed 40 µL sample loops)—with an Agilent 1290 Infinity II High Speed Pump for second-dimension chromatography. Diode array detection was deployed in both the first and the second dimension. In this example, the sample was an IgG standard purchased from Sigma. First-dimension method parameters are shown in Table 1. The second-dimension technique is shown in Table 2.
| Parameter | Description |
|---|---|
| Column | Agilent Bio-monolith Protein A column at room temperature |
| Flow rate | 0.75 mL/min |
| Mobile phase |
A) 100 mM PBS B) 500 mM acetic acid |
| Gradient |
0 minutes – 0 %B 0.5 minutes – 0 %B 0.51 minutes – 100 %B 2.5 minutes – 100 %B 2.6 minutes – 0 %B |
Table 1. First-dimension separation—Protein A affinity capture with UV detection.
| Parameter | Description |
|---|---|
| Column | Agilent AdvanceBio SEC 300A, 2.7 µm at 25 °C |
| Flow rate | 0.35 mL/min |
| Mobile phase | 10 mM PBS pH 7.4, isocratic |
| 2D-LC mode | Multiple heart-cutting |
Table 2. Second-dimension separation—Size exclusion chromatography.
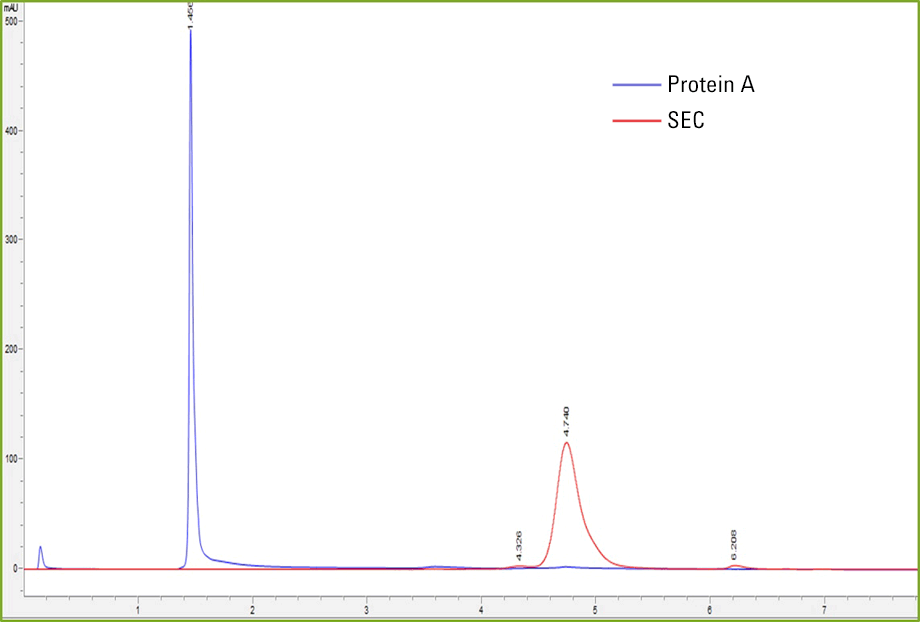
Figure 2. Protein A affinity capture and size exclusion separation of heat-stressed IgG.
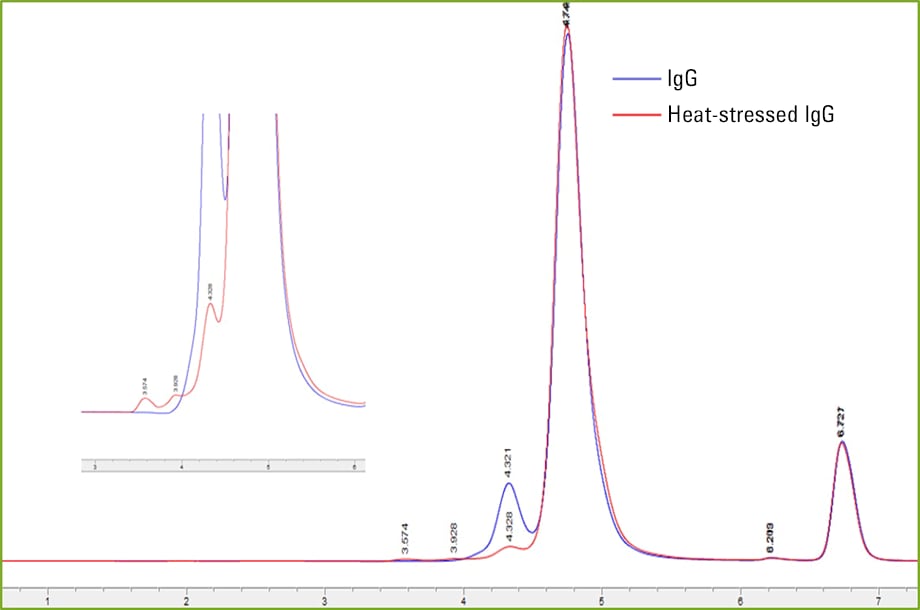
Figure 3. 2D size exclusion separation overlay of IgG and heat-stressed IgG.
2D separation technique saves time and increases efficiency
2D separation served to speed up the aggregate analysis of monoclonal antibodies. By combining two steps in the aggregation characterization, we were able to conduct the entire analysis with one injection, thus removing the fraction collection step all together.
After demonstrating the feasibility of coupling Protein A and SEC techniques together in one analysis with the Agilent 1290 Infinity II 2D-LC solution, we set out to test SEC detection of different aggregation concentrations using the 2D method. The IgG standard was heat stressed in a hot water bath for 24 hours at 65 °C. This resulted in a larger concentration of aggregates as shown in Figures 2 and 3. We can see clear resolution of aggregates from the monomer and dimer species in Figure 3.
To see the full study results, download and read Agilent Application Note 5991-7223EN.
Agilent 1290, LC columns, and AdvanceBio SEC combine to deliver versatile, quick analysis
The Agilent 1290 Infinity II 2D-LC solution offers a versatile and efficient way to determine mAb titer and aggregation in one single method, eliminating the need for fraction collection and multiple injections. Agilent Bio-monolith Protein A columns used as the first dimension of a 2D-LC workflow are well suited for the analytical-scale purification of mAbs for titer determination and as an initial purification step prior to aggregation analysis. The new Agilent AdvanceBio SEC 300Å column shows excellent resolution of aggregate species from monomer to enable accurate evaluation as the second dimension in a 2D-LC workflow.
Agilent offers a wide range of LC systems, columns, and supplies
Discover a wide range of LC systems, columns, and supplies designed to ensure the highest operational efficiency in your busy lab. Whether you are looking to increase sample throughput, accelerate method development, or solve challenging applications, Agilent’s highly modular approach provides the flexibility you need to excel. To learn more, contact your Agilent representative who can help you identify the right solutions for your particular application.
References
- Rapid Human Polyclonal IgG Quantification Using the Agilent Bio-Monolith Protein A HPLC Column, Agilent Technologies Application Note, publication number 5989-9733EN, 2008.
- Agilent Bio-Monolith Protein A Monitors Monoclonal Antibody Titer from Cell Cultures, Agilent Technologies Application Note, publication number 5991-2990EN, 2014.
For Research Use Only. Not for use in diagnostic procedures.
This information is subject to change without notice.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – September 2016
All articles in this issue
-
 High-resolution, efficient quantification of aggregates in protein therapeutics with Agilent 1290 Infinity II 2D-LC Solution
High-resolution, efficient quantification of aggregates in protein therapeutics with Agilent 1290 Infinity II 2D-LC Solution -
 Fast determination of bitter, clove-like flavor in beer with the Agilent 1290 Infinity II LC
Fast determination of bitter, clove-like flavor in beer with the Agilent 1290 Infinity II LC -
 Direct heating delivers a new paradigm in gas chromatography analysis
Direct heating delivers a new paradigm in gas chromatography analysis -
 Implementing an extractable and leachable (E&L) study from a pharmaceutical product by high resolution QTOF LC-MS
Implementing an extractable and leachable (E&L) study from a pharmaceutical product by high resolution QTOF LC-MS -
 Multi-omic analysis helps researchers to integrate complex omics data in cancer research
Multi-omic analysis helps researchers to integrate complex omics data in cancer research -
 Gain control—Agilent 490-PRO Micro GC optimizes process monitoring
Gain control—Agilent 490-PRO Micro GC optimizes process monitoring
Figure 2

Protein A affinity capture and size exclusion separation of heat-stressed IgG.
Figure 3

2D size exclusion separation overlay of IgG and heat-stressed IgG.